
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Drug/Regimen
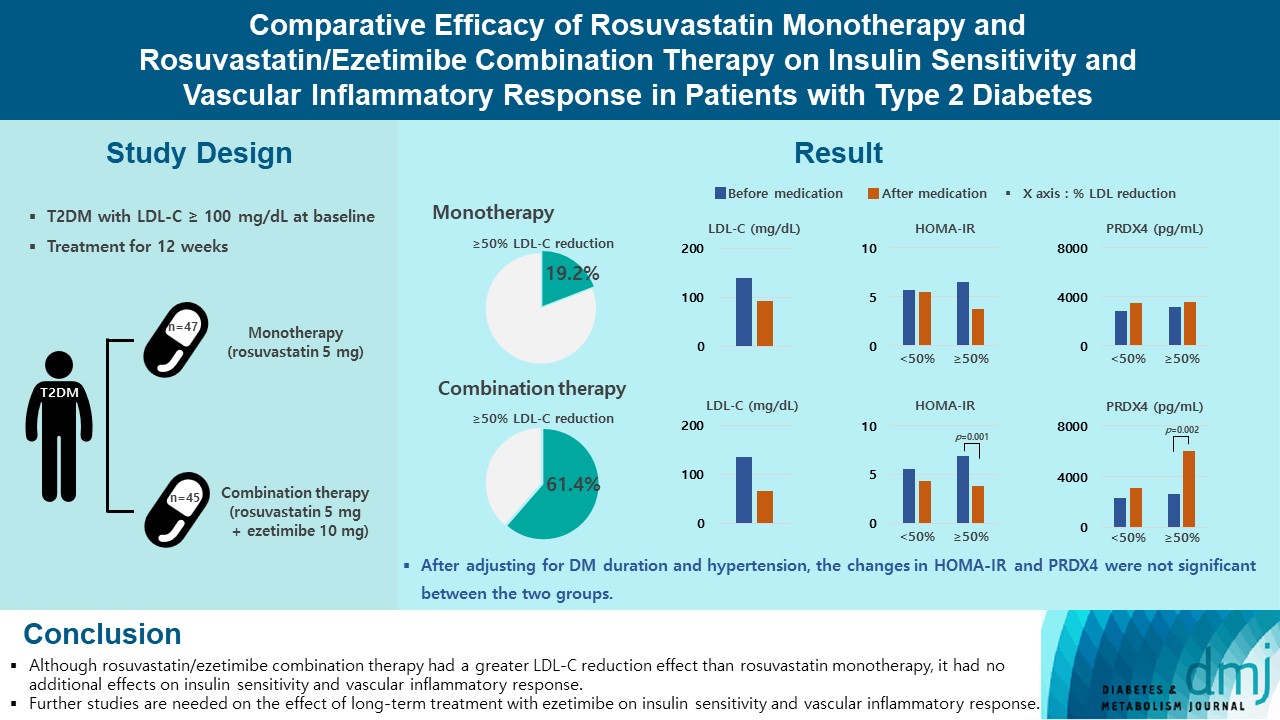
- Comparative Efficacy of Rosuvastatin Monotherapy and Rosuvastatin/Ezetimibe Combination Therapy on Insulin Sensitivity and Vascular Inflammatory Response in Patients with Type 2 Diabetes Mellitus
- Ji Hye Han, Kyong Hye Joung, Jun Choul Lee, Ok Soon Kim, Sorim Choung, Ji Min Kim, Yea Eun Kang, Hyon-Seung Yi, Ju Hee Lee, Bon Jeong Ku, Hyun Jin Kim
- Diabetes Metab J. 2024;48(1):112-121. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0402
- 2,037 View
- 223 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Type 2 diabetes mellitus (T2DM) induces endothelial dysfunction and inflammation, which are the main factors for atherosclerosis and cardiovascular disease. The present study aimed to compare the effects of rosuvastatin monotherapy and rosuvastatin/ezetimibe combination therapy on lipid profile, insulin sensitivity, and vascular inflammatory response in patients with T2DM.
Methods
A total of 101 patients with T2DM and dyslipidemia were randomized to either rosuvastatin monotherapy (5 mg/day, n=47) or rosuvastatin/ezetimibe combination therapy (5 mg/10 mg/day, n=45) and treated for 12 weeks. Serum lipids, glucose, insulin, soluble intercellular adhesion molecule-1 (sICAM-1), and peroxiredoxin 4 (PRDX4) levels were determined before and after 12 weeks of treatment.
Results
The reduction in low density lipoprotein cholesterol (LDL-C) by more than 50% from baseline after treatment was more in the combination therapy group. The serum sICAM-1 levels increased significantly in both groups, but there was no difference between the two groups. The significant changes in homeostasis model assessment of insulin resistance (HOMA-IR) and PRDX4 were confirmed only in the subgroup in which LDL-C was reduced by 50% or more in the combination therapy group. However, after adjusting for diabetes mellitus duration and hypertension, the changes in HOMA-IR and PRDX4 were not significant between the two groups.
Conclusion
Although rosuvastatin/ezetimibe combination therapy had a greater LDL-C reduction effect than rosuvastatin monotherapy, it had no additional effects on insulin sensitivity and vascular inflammatory response. Further studies are needed on the effect of long-term treatment with ezetimibe on insulin sensitivity and vascular inflammatory response. -
Citations
Citations to this article as recorded by- Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus
Eun Roh
Diabetes & Metabolism Journal.2024; 48(1): 55. CrossRef
- Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus
- Clinical Diabetes & Therapeutics
- Effectiveness and Safety of Adding Basal Insulin Glargine in Patients with Type 2 Diabetes Mellitus Exhibiting Inadequate Response to Metformin and DPP-4 Inhibitors with or without Sulfonylurea
- Yu Mi Kang, Chang Hee Jung, Seung-Hwan Lee, Sang-Wook Kim, Kee-Ho Song, Sin Gon Kim, Jae Hyeon Kim, Young Min Cho, Tae Sun Park, Bon Jeong Ku, Gwanpyo Koh, Dol Mi Kim, Byung-Wan Lee, Joong-Yeol Park
- Diabetes Metab J. 2019;43(4):432-446. Published online June 19, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0092
- 5,558 View
- 90 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background We aimed to investigate the effectiveness and safety of adding basal insulin to initiating dipeptidyl peptidase-4 (DPP-4) inhibitor and metformin and/or sulfonylurea (SU) in achieving the target glycosylated hemoglobin (HbA1c) in patients with type 2 diabetes mellitus (T2DM).
Methods This was a single-arm, multicenter, 24-week, open-label, phase 4 study in patients with inadequately controlled (HbA1c ≥7.5%) T2DM despite the use of DPP-4 inhibitor and metformin. A total of 108 patients received insulin glargine while continuing oral antidiabetic drugs (OADs). The primary efficacy endpoint was the percentage of subjects achieving HbA1c ≤7.0%. Other glycemic profiles were also evaluated, and the safety endpoints were adverse events (AEs) and hypoglycemia.
Results The median HbA1c at baseline (8.9%; range, 7.5% to 11.1%) decreased to 7.6% (5.5% to 11.7%) at 24 weeks. Overall, 31.7% subjects (
n =33) achieved the target HbA1c level of ≤7.0%. The mean differences in body weight and fasting plasma glucose were 1.2±3.4 kg and 56.0±49.8 mg/dL, respectively. Hypoglycemia was reported in 36 subjects (33.3%, 112 episodes), all of which were fully recovered. There was no serious AE attributed to insulin glargine. Body weight change was significantly different between SU users and nonusers (1.5±2.5 kg vs. −0.9±6.0 kg,P =0.011).Conclusion The combination add-on therapy of insulin glargine, on metformin and DPP-4 inhibitors with or without SU was safe and efficient in reducing HbA1c levels and thus, is a preferable option in managing T2DM patients exhibiting dysglycemia despite the use of OADs.
-
Citations
Citations to this article as recorded by- Glycaemic control with add‐on thiazolidinedione or a sodium‐glucose co‐transporter‐2 inhibitor in patients with type 2 diabetes after the failure of an oral triple antidiabetic regimen: A 24‐week, randomized controlled trial
Jaehyun Bae, Ji Hye Huh, Minyoung Lee, Yong‐Ho Lee, Byung‐Wan Lee
Diabetes, Obesity and Metabolism.2021; 23(2): 609. CrossRef - Beneficial effect of anti-diabetic drugs for nonalcoholic fatty liver disease
Kyung-Soo Kim, Byung-Wan Lee
Clinical and Molecular Hepatology.2020; 26(4): 430. CrossRef
- Glycaemic control with add‐on thiazolidinedione or a sodium‐glucose co‐transporter‐2 inhibitor in patients with type 2 diabetes after the failure of an oral triple antidiabetic regimen: A 24‐week, randomized controlled trial
- Others
- Serum R-Spondin 1 Is a New Surrogate Marker for Obesity and Insulin Resistance
- Yea Eun Kang, Ji Min Kim, Hyon-Seung Yi, Kyong Hye Joung, Ju Hee Lee, Hyun Jin Kim, Bon Jeong Ku
- Diabetes Metab J. 2019;43(3):368-376. Published online October 23, 2018
- DOI: https://doi.org/10.4093/dmj.2018.0066
- 5,009 View
- 75 Download
- 6 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Recent
in vivo studies indicated that R-spondin 1 (RSPO1) regulates food intake and increases insulin secretion, but its role in humans remains unknown. This study investigated the association between serum levels of RSPO1 and diverse metabolic parameters in humans.Methods The study population consisted of 43 subjects with newly diagnosed diabetes mellitus, and 79 non-diabetic participants. Serum levels of RSPO1 were measured using the enzyme-linked immunosorbent assay. The relationships between circulating RSPO1 and diverse metabolic parameters were analyzed.
Results Circulating RSPO1 levels increased to a greater extent in the obese group than in the lean group. Moreover, serum levels of RSPO1 were higher in the insulin-resistant group than in the insulin-sensitive group. Serum levels of RSPO1 were significantly correlated with a range of metabolic parameters including body mass index, fasting C-peptide, homeostasis model assessment of insulin resistance index, and lipid profile. Moreover, levels were significantly associated with insulin resistance and obesity in non-diabetic subjects.
Conclusion This study demonstrated the association between serum levels of RSPO1 and a range of metabolic parameters in humans. Serum levels of RSPO1 are significantly related to obesity and insulin resistance, although the precise mechanisms remain unknown.
-
Citations
Citations to this article as recorded by- LGR4: A New Receptor Member in Endocrine and Metabolic Diseases
Ningning Zhang, Mingyang Yuan, Jiqiu Wang
Endocrine Reviews.2023; 44(4): 647. CrossRef - R-Spondin1 and tumor necrosis factor-alpha in infertile women with polycystic ovary syndrome: relationships with insulin resistance and other parameters
Tuğba GÜRBÜZ, Oya GÖKMEN, Asena AYAR MADENLİ, Berna DİLBAZ
Journal of Health Sciences and Medicine.2023; 6(2): 449. CrossRef - An early prediction model for type 2 diabetes mellitus based on genetic variants and nongenetic risk factors in a Han Chinese cohort
Jinjin Li, Qun Ye, Hongxiao Jiao, Wanyao Wang, Kai Zhang, Chen Chen, Yuan Zhang, Shuzhi Feng, Ximo Wang, Yubao Chen, Huailin Gao, Fengjiang Wei, Wei-Dong Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Emerging Therapeutic Strategies for Attenuating Tubular EMT and Kidney Fibrosis by Targeting Wnt/β-Catenin Signaling
Lichao Hu, Mengyuan Ding, Weichun He
Frontiers in Pharmacology.2022;[Epub] CrossRef - Does Serum R-Spondin-1 Play a Role in PCOS Pathophysiology?
Osman BAŞPINAR, Yasin ŞİMŞEK, Derya KOÇER, Oğuzhan Sıtkı DİZDAR, Hatice KAYIŞ TOPALOĞLU
Genel Tıp Dergisi.2022; 32(5): 490. CrossRef - Silencing of RSPO1 mitigates obesity-related renal fibrosis in mice by deactivating Wnt/β-catenin pathway
Xuesong Su, Guangyu Zhou, Mi Tian, Si Wu, Yanqiu Wang
Experimental Cell Research.2021; 405(2): 112713. CrossRef - Exosome miR‐27a‐3p secreted from adipocytes targets ICOS to promote antitumor immunity in lung adenocarcinoma
Xuehan Fan, Jingya Wang, Tingting Qin, Yujia Zhang, Wenting Liu, Kaiting Jiang, Dingzhi Huang
Thoracic Cancer.2020; 11(6): 1453. CrossRef - Integrative Analyses of Genes Associated with Subcutaneous Insulin Resistance
Manoj Kumar Pujar, Basavaraj Vastrad, Chanabasayya Vastrad
Biomolecules.2019; 9(2): 37. CrossRef
- LGR4: A New Receptor Member in Endocrine and Metabolic Diseases
- Others
- Serum Soluble Epidermal Growth Factor Receptor Level Increase in Patients Newly Diagnosed with Type 2 Diabetes Mellitus
- Ji Min Kim, Sorim Choung, Kyong Hye Joung, Ju Hee Lee, Hyun Jin Kim, Bon Jeong Ku
- Diabetes Metab J. 2018;42(4):343-347. Published online May 2, 2018
- DOI: https://doi.org/10.4093/dmj.2017.0082
- 4,319 View
- 50 Download
- 6 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader We analyzed circulating soluble epidermal growth factor receptor (sEGFR) levels in humans. Serum sEGFR levels were higher in subjects with newly diagnosed type 2 diabetes mellitus compared with controls. Serum sEGFR was positively correlated with glycosylated hemoglobin and serum glucose and negatively correlated with serum insulin and C-peptide levels.
-
Citations
Citations to this article as recorded by- Increased serum extrachromosomal circular DNA SORBS1circle level is associated with insulin resistance in patients with newly diagnosed type 2 diabetes mellitus
Xiang Kong, Shu-jun Wan, Tian-bing Chen, Lan Jiang, Yu-jie Xing, Ya-ping Bai, Qiang Hua, Xin-ming Yao, Yong-li Zhao, Hong-mei Zhang, De-guo Wang, Qing Su, Kun Lv
Cellular & Molecular Biology Letters.2024;[Epub] CrossRef - A Pilot Genome-Wide Association Study Identifies Novel Markers of Metabolic Syndrome in Patients with Psoriasis
Seung-Min Oh, Su-Kang Kim, Hye-Jin Ahn, Ki-Heon Jeong
Annals of Dermatology.2023; 35(4): 285. CrossRef - Effect of cholesterol-lowering agents on soluble epidermal growth factor receptor level in type 2 diabetes and hypercholesterolemia
Jun Choul Lee, Kyong Hye Joung, Ji Min Kim, Seon Mee Kang, Hyun Jin Kim, Bon Jeong Ku
Medicine.2022; 101(34): e30287. CrossRef - Soluble EGFR, a hepatokine, and adipsin, an adipokine, are biomarkers correlated with distinct aspects of insulin resistance in type 2 diabetes subjects
Mayu Kyohara, Jun Shirakawa, Tomoko Okuyama, Yu Togashi, Ryota Inoue, Jinghe Li, Daisuke Miyashita, Yasuo Terauchi
Diabetology & Metabolic Syndrome.2020;[Epub] CrossRef - Epidermal growth factor protects against myocardial ischaemia reperfusion injury through activating Nrf2 signalling pathway
Jun Ma, Ge Jin
Free Radical Research.2019; 53(3): 313. CrossRef
- Increased serum extrachromosomal circular DNA SORBS1circle level is associated with insulin resistance in patients with newly diagnosed type 2 diabetes mellitus
- Others
- Clinical Implications of Using Post-Challenge Plasma Glucose Levels for Early Diagnosis of Type 2 Diabetes Mellitus in Older Individuals
- Kyong Hye Joung, Sang Hyun Ju, Ji Min Kim, Sorim Choung, Jae Min Lee, Kang Seo Park, Hyun Jin Kim, Bon Jeong Ku
- Diabetes Metab J. 2018;42(2):147-154. Published online February 13, 2018
- DOI: https://doi.org/10.4093/dmj.2018.42.2.147
- 4,645 View
- 36 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The aim of this study was to explore the differences in the clinical characteristics and diagnostic rates of diabetes mellitus (DM) according to various criteria in different age groups and to evaluate the efficacy of each criterion for screening older patients.
Methods We studied 515 patients and measured the fasting plasma glucose level (FPG), 2-hour plasma glucose level after the 75 g oral glucose tolerance test (2-hour postload glucose [2-h PG]), and glycosylated hemoglobin (HbA1c) for re-evaluation of hyperglycemia without a history of diabetes. Patients with newly diagnosed DM were grouped by age as younger (<65 years) or older (≥65 years).
Results Older patients had significantly lower HbA1c, FPG, and 2-h PG levels and a higher homeostatic level of pancreatic β-cell function compared with younger patients (
P <0.001). The older group had the lowest diagnostic rate when using the FPG level (45.5%) and the highest diagnostic rate when using the 2-h PG level (84.6%). These results were mostly due to the higher frequency of isolated post-challenge hyperglycemia in the older patients than in the younger group (28.8% vs. 9.2%). The use of both the FPG and HbA1c levels significantly enhanced the low diagnostic power when employing only the FPG levels in the older group (71.2% vs. 45.5%).Conclusion In the older patients, the 2-h PG level was the most accurate diagnostic criterion. When we consider the costs and convenience, a combination of the FPG and HbA1c criteria may be recommended as a screening test for DM in older people.
-
Citations
Citations to this article as recorded by- International Diabetes Federation Position Statement on the 1-hour post-load plasma glucose for the diagnosis of intermediate hyperglycaemia and type 2 diabetes
Michael Bergman, Melania Manco, Ilhan Satman, Juliana Chan, Maria Inês Schmidt, Giorgio Sesti, Teresa Vanessa Fiorentino, Muhammad Abdul-Ghani, Ram Jagannathan, Pramod Kumar Thyparambil Aravindakshan, Rafael Gabriel, Viswanathan Mohan, Martin Buysschaert,
Diabetes Research and Clinical Practice.2024; 209: 111589. CrossRef - A unified technique for entropy enhancement based diabetic retinopathy detection using hybrid neural network
Fatima, Muhammad Imran, Anayat Ullah, Muhammad Arif, Rida Noor
Computers in Biology and Medicine.2022; 145: 105424. CrossRef - In-silico identification of peroxisome proliferator-activated receptor (PPAR)α/γ agonists from Ligand Expo Components database
Xiao-Yan Feng, Ting-Ting Ding, Ya-Ya Liu, Wei-Ren Xu, Xian-Chao Cheng
Journal of Biomolecular Structure and Dynamics.2021; 39(5): 1853. CrossRef
- International Diabetes Federation Position Statement on the 1-hour post-load plasma glucose for the diagnosis of intermediate hyperglycaemia and type 2 diabetes
- Others
- Addition of Ipragliflozin to Metformin Treatment in Korean Patients with Type 2 Diabetes Mellitus: Subgroup Analysis of a Phase 3 Trial
- Kyung-Wan Min, Bon Jeong Ku, Ji-Hyun Lee, Min-Seon Kim, Kyu-Jeung Ahn, Moon-Kyu Lee, Satoshi Kokubo, Satoshi Yoshida, Hyun-Ji Cho, Bong-Soo Cha
- Diabetes Metab J. 2017;41(2):135-145. Published online January 11, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.2.135
- 4,959 View
- 59 Download
- 14 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background This is a subgroup analysis of Korean patients from a phase 3 clinical trial investigating the efficacy and safety of ipragliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin.
Methods This multicenter, placebo-controlled, double-blind, parallel-group study was carried out between November 2011 and January 2013. Patients entered a 2-week placebo pretreatment period, followed by a 24-week treatment period with either ipragliflozin (50 mg/day) or placebo, while continuing metformin. Efficacy outcomes (glycosylated hemoglobin [HbA1c], fasting plasma glucose [FPG], and body weight) and safety outcomes (treatment-emergent adverse events [TEAEs]) were measured and compared between the two treatment groups for patients enrolled in all 18 study sites in Korea.
Results Eighty-two Korean patients received ipragliflozin (
n =43) or placebo (n =39) during the study period. Mean changes in HbA1c levels from baseline to the end of treatment were –0.97% in the ipragliflozin group and –0.31% in the placebo group, with an adjusted between-group difference of –0.60% (P <0.001). Compared to placebo, FPG and body weight also decreased significantly (bothP <0.001) from baseline after treatment in the ipragliflozin group, with between-group differences of –21.4 mg/dL and –1.53 kg, respectively. Decreased weight was the most common TEAE in the ipragliflozin group (7.0%); there were no reports of genital and urinary tract infection.Conclusion Ipragliflozin treatment in addition to metformin led to significant improvement in glycemic outcomes and reduction in body weight in Korean patients with type 2 diabetes mellitus, compared with metformin treatment alone; the safety profile was comparable in both groups.
-
Citations
Citations to this article as recorded by- Add-on therapy with dapagliflozin in routine outpatient care of type 2 diabetes patients from Turkey: a retrospective cohort study on HbA1c, body weight, and blood pressure outcomes
Derun Taner Ertugrul, Erdal Kan, Cigdem Bahadir Tura, Haci Bayram Tugtekin, Hayati Ayakta, Mehmet Celebioglu, Ceren Yılmaz, Onur Utebay, Ilhan Yetkin, Eren Gurkan, Kerem Sezer, Ramazan Gen, Suleyman Ozcaylak, Yildiz Okuturlar, Mehmet Coskun, Nilgun Govec
International Journal of Diabetes in Developing Countries.2022; 42(1): 147. CrossRef SGLT2 Inhibitors as Add-On Therapy to Metformin for People with Type 2 Diabetes: A Review of Placebo-Controlled Trials in Asian versus Non-Asian Patients
André J Scheen
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2020; Volume 13: 2765. CrossRef- Ipragliflozin Additively Ameliorates Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Controlled with Metformin and Pioglitazone: A 24-Week Randomized Controlled Trial
Eugene Han, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha
Journal of Clinical Medicine.2020; 9(1): 259. CrossRef - Safety of Ipragliflozin in Patients with Type 2 Diabetes Mellitus: Pooled Analysis of Phase II/III/IV Clinical Trials
Atsunori Kashiwagi, Marina V. Shestakova, Yuichiro Ito, Masahiro Noguchi, Wim Wilpshaar, Satoshi Yoshida, John P. H. Wilding
Diabetes Therapy.2019; 10(6): 2201. CrossRef - Mechanistic effects of SGLT2 inhibition on blood pressure in diabetes
Habib Yaribeygi, Stephen L. Atkin, Amirhossein Sahebkar
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(2): 1679. CrossRef - Ipragliflozin as an add-on therapy in type 2 diabetes mellitus patients: An evidence-based pharmacoeconomics evaluation
Hongmei Wang, Gaoqiong Yao, Xi Chen, Jing Ouyang, Jiadan Yang
Diabetes Research and Clinical Practice.2019; 157: 107867. CrossRef - Characteristics of Dapagliflozin Responders: A Longitudinal, Prospective, Nationwide Dapagliflozin Surveillance Study in Korea
Eugene Han, Ari Kim, Sung Jae Lee, Je-Yon Kim, Jae Hyeon Kim, Woo Je Lee, Byung-Wan Lee
Diabetes Therapy.2018; 9(4): 1689. CrossRef - A phase 3 randomized placebo-controlled trial to assess the efficacy and safety of ipragliflozin as an add-on therapy to metformin in Russian patients with inadequately controlled type 2 diabetes mellitus
Marina V. Shestakova, John P.H. Wilding, Wim Wilpshaar, Reiner Tretter, Valeria L. Orlova, Andrey F. Verbovoy
Diabetes Research and Clinical Practice.2018; 146: 240. CrossRef - Efficacy and safety of ipragliflozin as an add‐on therapy to sitagliptin and metformin in Korean patients with inadequately controlled type 2 diabetes mellitus: A randomized controlled trial
Kyung‐Ah Han, Suk Chon, Choon Hee Chung, Soo Lim, Kwan‐Woo Lee, SeiHyun Baik, Chang Hee Jung, Dong‐Sun Kim, Kyong Soo Park, Kun‐Ho Yoon, In‐Kyu Lee, Bong‐Soo Cha, Taishi Sakatani, Sumi Park, Moon‐Kyu Lee
Diabetes, Obesity and Metabolism.2018; 20(10): 2408. CrossRef - Antihyperglycemic Agent Therapy for Adult Patients with Type 2 Diabetes Mellitus 2017: A Position Statement of the Korean Diabetes Association
Seung-Hyun Ko, Kyu-Yeon Hur, Sang Youl Rhee, Nan-Hee Kim, Min Kyong Moon, Seok-O Park, Byung-Wan Lee, Hyun Jin Kim, Kyung Mook Choi, Jin Hwa Kim
Diabetes & Metabolism Journal.2017; 41(5): 337. CrossRef - Antihyperglycemic agent therapy for adult patients with type 2 diabetes mellitus 2017: a position statement of the Korean Diabetes Association
Seung-Hyun Ko, Kyu-Yeon Hur, Sang Youl Rhee, Nan-Hee Kim, Min Kyong Moon, Seok-O Park, Byung-Wan Lee, Hyun Jin Kim, Kyung Mook Choi, Jin Hwa Kim
The Korean Journal of Internal Medicine.2017; 32(6): 947. CrossRef - Combination therapy of oral hypoglycemic agents in patients with type 2 diabetes mellitus
Min Kyong Moon, Kyu Yeon Hur, Seung-Hyun Ko, Seok-O Park, Byung-Wan Lee, Jin Hwa Kim, Sang Youl Rhee, Hyun Jin Kim, Kyung Mook Choi, Nan-Hee Kim
The Korean Journal of Internal Medicine.2017; 32(6): 974. CrossRef - Combination Therapy of Oral Hypoglycemic Agents in Patients with Type 2 Diabetes Mellitus
Min Kyong Moon, Kyu-Yeon Hur, Seung-Hyun Ko, Seok-O Park, Byung-Wan Lee, Jin Hwa Kim, Sang Youl Rhee, Hyun Jin Kim, Kyung Mook Choi, Nan-Hee Kim
Diabetes & Metabolism Journal.2017; 41(5): 357. CrossRef
- Add-on therapy with dapagliflozin in routine outpatient care of type 2 diabetes patients from Turkey: a retrospective cohort study on HbA1c, body weight, and blood pressure outcomes
- Others
- Effect of Atorvastatin on Growth Differentiation Factor-15 in Patients with Type 2 Diabetes Mellitus and Dyslipidemia
- Ji Min Kim, Min Kyung Back, Hyon-Seung Yi, Kyong Hye Joung, Hyun Jin Kim, Bon Jeong Ku
- Diabetes Metab J. 2016;40(1):70-78. Published online February 19, 2016
- DOI: https://doi.org/10.4093/dmj.2016.40.1.70
- 4,173 View
- 33 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Elevated serum levels of growth differentiation factor-15 (GDF-15) are associated with type 2 diabetes. Therefore, the effects of atorvastatin on metabolic parameters and GDF-15 levels in patients with type 2 diabetes and dyslipidemia were evaluated.
Methods In this prospective randomized trial from February 2013 to March 2014, 50 consecutive type 2 diabetic patients with a low density lipoprotein cholesterol (LDL-C) levels ≥100 mg/dL were enrolled. The patients were divided into two groups based on the amount of atorvastatin prescribed, 10 mg/day (
n =23) or 40 mg/day (n =27). The effect of atorvastatin on metabolic parameters, including lipid profiles and GDF-15 levels, at baseline and after 8 weeks of treatment were compared.Results The baseline metabolic parameters and GDF-15 levels were not significantly different between the two groups. After 8 weeks of treatment, the total cholesterol (TC) and LDL-C levels were significantly decreased in both groups. The mean changes in TC and LDL-C levels were more significant in the 40 mg atorvastatin group. The GDF-15 level was decreased in the 10 mg atorvastatin group, from 1,460.6±874.8 to 1,451.0±770.8 pg/mL, and was increased in the 40 mg atorvastatin group, from 1,271.6±801.0 to 1,341.4±855.2 pg/mL. However, the change in the GDF-15 level was not statistically significant in the 10 or 40 mg atorvastatin group (
P =0.665 andP =0.745, respectively).Conclusion The GDF-15 levels were not significantly changed after an 8-week treatment with atorvastatin in type 2 diabetic patients.
-
Citations
Citations to this article as recorded by- The relationship of Growth differentiation factor-15 with renal damage and dyslipidemia in non-albuminuric and albuminuric Type-2 Diabetes Mellitus
Hasan Esat Yücel, Bilal İlanbey
Medical Science and Discovery.2022; 9(6): 334. CrossRef - Comparative effectiveness of statins on non-high density lipoprotein cholesterol in people with diabetes and at risk of cardiovascular disease: systematic review and network meta-analysis
Alexander Hodkinson, Dialechti Tsimpida, Evangelos Kontopantelis, Martin K Rutter, Mamas A Mamas, Maria Panagioti
BMJ.2022; : e067731. CrossRef - The Cytokine Growth Differentiation Factor-15 and Skeletal Muscle Health: Portrait of an Emerging Widely Applicable Disease Biomarker
Boel De Paepe
International Journal of Molecular Sciences.2022; 23(21): 13180. CrossRef - Biomarkers of subclinical atherosclerosis in patients with psoriasis
Hannah Kaiser, Xing Wang, Amanda Kvist-Hansen, Martin Krakauer, Peter Michael Gørtz, Benjamin D. McCauley, Lone Skov, Christine Becker, Peter Riis Hansen
Scientific Reports.2021;[Epub] CrossRef - Growth differentiation factor-15 regulates oxLDL-induced lipid homeostasis and autophagy in human macrophages
Kathrin Ackermann, Gabriel A. Bonaterra, Ralf Kinscherf, Anja Schwarz
Atherosclerosis.2019; 281: 128. CrossRef
- The relationship of Growth differentiation factor-15 with renal damage and dyslipidemia in non-albuminuric and albuminuric Type-2 Diabetes Mellitus
- Response: GDF15 Is a Novel Biomarker for Impaired Fasting Glucose (
Diabetes Metab J 2014;38:472-9) - Jun Hwa Hong, Bon Jeong Ku, Minho Shong
- Diabetes Metab J. 2015;39(1):84-86. Published online February 16, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.1.84
- 2,910 View
- 27 Download
- GDF15 Is a Novel Biomarker for Impaired Fasting Glucose
- Jun Hwa Hong, Hyo Kyun Chung, Hye Yoon Park, Kyong-Hye Joung, Ju Hee Lee, Jin Gyu Jung, Koon Soon Kim, Hyun Jin Kim, Bon Jeong Ku, Minho Shong
- Diabetes Metab J. 2014;38(6):472-479. Published online December 15, 2014
- DOI: https://doi.org/10.4093/dmj.2014.38.6.472
- 5,487 View
- 75 Download
- 67 Web of Science
- 63 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Growth differentiation factor-15 (GDF15) is a protein that belongs to the transforming growth factor β superfamily. An elevated serum level of GDF15 was found to be associated with type 2 diabetes mellitus (T2DM). T2DM is an inflammatory disease that progresses from normal glucose tolerance (NGT) to impaired fasting glucose (IFG). Hence, we aimed to validate the relationship between GDF15 and IFG.
Methods The participants were divided into the following three groups: NGT (
n =137), IFG (n =29), and T2DM (n =75). The controls and T2DM outpatients visited the hospital for routine health check-ups. We used fasting blood glucose to detect IFG in nondiabetic patients. We checked the body mass index (BMI), C-reactive protein level, metabolic parameters, and fasting serum GDF15 level.Results Age, BMI, triglyceride, insulin, glucose, homeostatic model assessment-insulin resistance (HOMA-IR), and GDF15 levels were elevated in the IFG and T2DM groups compared to the NGT group. In the correlation analysis between metabolic parameters and GDF15, age and HOMA-IR had a significant positive correlation with GDF15 levels. GDF15 significantly discriminated between IFG and NGT, independent of age, BMI, and HOMA-IR. The serum levels of GDF15 were more elevated in men than in women. As a biomarker for IFG based on the receiver operating characteristic curve analysis, the cutoff value of GDF15 was 510 pg/mL in males and 400 pg/mL in females.
Conclusion GDF15 had a positive correlation with IR independent of age and BMI, and the serum level of GDF15 was increased in the IFG and T2DM groups. GDF15 may be a novel biomarker for detecting IFG in nondiabetic patients.
-
Citations
Citations to this article as recorded by- Effect of a 6-Week Carbohydrate-Reduced High-Protein Diet on Levels of FGF21 and GDF15 in People With Type 2 Diabetes
Michael M Richter, Mads N Thomsen, Mads J Skytte, Sasha A S Kjeldsen, Amirsalar Samkani, Jan Frystyk, Faidon Magkos, Jens J Holst, Sten Madsbad, Thure Krarup, Steen B Haugaard, Nicolai J Wewer Albrechtsen
Journal of the Endocrine Society.2024;[Epub] CrossRef - Serum Growth Differentiation Factor 15 Levels Predict the Incidence of Frailty among Patients with Cardiometabolic Diseases
Kazuhito Oba, Joji Ishikawa, Yoshiaki Tamura, Yasunori Fujita, Masafumi Ito, Ai Iizuka, Yoshinori Fujiwara, Remi Kodera, Kenji Toyoshima, Yuko Chiba, Masashi Tanaka, Atsushi Araki
Gerontology.2024; : 1. CrossRef - Growth differentiation factor 15 and malnutrition in older adults
Nazanin Rostami, Blanca Fabre-Estremera, Antonio Buño-Soto, José R Banegas, Fernando Rodríguez-Artalejo, Rosario Ortolá
The Journal of nutrition, health and aging.2024; 28(6): 100230. CrossRef - Evaluation of the relation between subclinical systolic dysfunction defined by four-dimensional speckle-tracking echocardiography and growth differentiation factor-15 levels in patients with acromegaly
Busra Firlatan, Ugur Nadir Karakulak, Vedat Hekimsoy, Burcin Gonul Iremli, Incilay Lay, Deniz Yuce, Selcuk Dagdelen, Giray Kabakci, Tomris Erbas
Hormones.2024;[Epub] CrossRef - Exploration of meteorin-like peptide (metrnl) predictors in type 2 diabetic patients: the potential role of irisin, and other biochemical parameters
Yaser Khajebishak, Amir Hossein Faghfouri, Ali Soleimani, Sadra Madani, Laleh Payahoo
Hormone Molecular Biology and Clinical Investigation.2023; 44(2): 127. CrossRef - Relationship between meteorin-like peptide (Metrnl) serum levels and inflammatory cytokines, oxidative stress biomarkers and body composition parameters in type 2 diabetes patients
Yaser Khajebishak, Sadra Madani, Amir Hossein Faghfouri, Ali Soleimani, Sara Ilaei, Said Peyrovi, Laleh Payahoo
Nutrition & Food Science.2023; 53(5): 861. CrossRef - Effects of acute exercise and exercise training on plasma GDF15 concentrations and associations with appetite and cardiometabolic health in individuals with overweight or obesity – A secondary analysis of a randomized controlled trial
Jonas Salling Quist, Anders Bue Klein, Kristine Færch, Kristine Beaulieu, Mads Rosenkilde, Anne Sofie Gram, Anders Sjödin, Signe Torekov, Bente Stallknecht, Christoffer Clemmensen, Martin Bæk Blond
Appetite.2023; 182: 106423. CrossRef - Significant increase of serum extracellular vesicle-packaged growth differentiation factor 15 in type 2 diabetes mellitus: a cross-sectional study
Wen Zhao, Xinwei Li, Xinxin Li, Lu Peng, Yu Li, Yunhui Du, Jianxun He, Yanwen Qin, Huina Zhang
European Journal of Medical Research.2023;[Epub] CrossRef - Association between growth differentiation factor 15 levels and gestational diabetes mellitus: A combined analysis
Yi-Cheng Lu, Song-Liang Liu, Yu-Shan Zhang, Fei Liang, Xiao-Yan Zhu, Yue Xiao, Jing Wang, Cong Ding, Sudipta Banerjee, Jie-Yun Yin, Qiu-Ping Ma
Frontiers in Endocrinology.2023;[Epub] CrossRef - Metformin triggers a kidney GDF15-dependent area postrema axis to regulate food intake and body weight
Song-Yang Zhang, Kyla Bruce, Zahra Danaei, Rosa J.W. Li, Daniel R. Barros, Rachel Kuah, Yu-Mi Lim, Laura H. Mariani, David Z. Cherney, Jennifer F.M. Chiu, Heather N. Reich, Tony K.T. Lam
Cell Metabolism.2023; 35(5): 875. CrossRef - Growth differentiation factor 15 (GDF-15) in endocrinology
Pedro Iglesias, Ramona A. Silvestre, Juan J. Díez
Endocrine.2023; 81(3): 419. CrossRef - Identification of biomarkers for glycaemic deterioration in type 2 diabetes
Roderick C. Slieker, Louise A. Donnelly, Elina Akalestou, Livia Lopez-Noriega, Rana Melhem, Ayşim Güneş, Frederic Abou Azar, Alexander Efanov, Eleni Georgiadou, Hermine Muniangi-Muhitu, Mahsa Sheikh, Giuseppe N. Giordano, Mikael Åkerlund, Emma Ahlqvist, A
Nature Communications.2023;[Epub] CrossRef - Metabolic syndrome and Growth Differentiation Factor 15 in older adults
Adrián Carballo-Casla, Esther García-Esquinas, Antonio Buño-Soto, Ellen A. Struijk, Esther López-García, Fernando Rodríguez-Artalejo, Rosario Ortolá
GeroScience.2022; 44(2): 867. CrossRef - Associations between GDF15 Levels and Pre-Diabetes in Non-Obese Subjects
Hao-Chang Hung, Hung-Tsung Wu, Ching-Han Lin, Hsuan-Wen Chou, Horng-Yih Ou, Chih-Jen Chang
Journal of Investigative Medicine.2022; 70(1): 79. CrossRef - Growth Differentiation Factor-15 as a Biomarker of Obese Pre-diabetes and Type 2 Diabetes Mellitus in Indian Subjects: A Case-control Study
Dipayan Roy, Purvi Purohit, Anupama Modi, Manoj Khokhar, Ravindra Kumar Gayaprasad Shukla, Ramkaran Chaudhary, Shrimanjunath Sankanagoudar, Praveen Sharma
Current Diabetes Reviews.2022;[Epub] CrossRef - Growth Differentiation Factor 15 Protects SH-SY5Y Cells From Rotenone-Induced Toxicity by Suppressing Mitochondrial Apoptosis
Peizheng Li, Hongbo Lv, Bohan Zhang, Ruonan Duan, Xiufang Zhang, Pengfei Lin, Chengyuan Song, Yiming Liu
Frontiers in Aging Neuroscience.2022;[Epub] CrossRef - GDF-15 as a Therapeutic Target of Diabetic Complications Increases the Risk of Gallstone Disease: Mendelian Randomization and Polygenic Risk Score Analysis
Lili Yu, Yajing Zhou, Lijuan Wang, Xuan Zhou, Jing Sun, Jiarui Xiao, Xiaolin Xu, Susanna C. Larsson, Shuai Yuan, Xue Li
Frontiers in Genetics.2022;[Epub] CrossRef - Sex-specific modulation of circulating growth differentiation factor-15 in patients with type 2 diabetes and/or obesity
Mohamed Asrih, Flore Sinturel, Richard Dubos, Idris Guessous, Zoltan Pataky, Charna Dibner, François R Jornayvaz, Karim Gariani
Endocrine Connections.2022;[Epub] CrossRef - Raised circulating soluble growth differentiation factor 15 is negatively associated with testosterone level in hypogonadic men with type 2 diabetes
Yufeng Mei, Yongnan Lyu, Juan Le, Di Li, Hang Liu, Zhiming Zhao, Yan Li
Diabetes/Metabolism Research and Reviews.2022;[Epub] CrossRef - NCAM1 and GDF15 are biomarkers of Charcot-Marie-Tooth disease in patients and mice
Matthew J Jennings, Alexia Kagiava, Leen Vendredy, Emily L Spaulding, Marina Stavrou, Denisa Hathazi, Anika Grüneboom, Vicky De Winter, Burkhard Gess, Ulrike Schara, Oksana Pogoryelova, Hanns Lochmüller, Christoph H Borchers, Andreas Roos, Robert W Burges
Brain.2022; 145(11): 3999. CrossRef - Metformin and growth differentiation factor 15 (GDF15) in type 2 diabetes mellitus: A hidden treasure
Hayder M. Al‐kuraishy, Ali I. Al‐Gareeb, Athanasios Alexiou, Marios Papadakis, Eman Hassan Nadwa, Sarah M. Albogami, Mohammed Alorabi, Hebatallah M. Saad, Gaber El‐Saber Batiha
Journal of Diabetes.2022; 14(12): 806. CrossRef - GDF15 is an exercise-induced hepatokine regulated by glucagon and insulin in humans
Peter Plomgaard, Jakob S. Hansen, Logan K. Townsend, Anders Gudiksen, Niels H. Secher, Jens O. Clemmesen, Rene K. Støving, Jens P. Goetze, David C. Wright, Henriette Pilegaard
Frontiers in Endocrinology.2022;[Epub] CrossRef - A study of serum growth differentiation factor 15 in Indian women with and without gestational diabetes mellitus in the third trimester of pregnancy and its association with pro-inflammatory markers and glucose metabolism
Sudipta Banerjee, Rana Bhattacharjee, Amitabh Sur, Pieu Adhikary, Subhankar Chowdhury
Diabetology International.2021; 12(3): 254. CrossRef - Growth Differentiation Factor 15 is a Cancer Cell-Induced Mitokine That Primes Thyroid Cancer Cells for Invasiveness
Yea Eun Kang, Jin Man Kim, Mi Ae Lim, Seong Eun Lee, Shinae Yi, Jung Tae Kim, Chan Oh, Lihua Liu, Yanli Jin, Seung-Nam Jung, Ho-Ryun Won, Jae Won Chang, Jeong Ho Lee, Hyun Jung Kim, Hyun Yong Koh, Sangmi Jun, Sun Wook Cho, Minho Shong, Bon Seok Koo
Thyroid.2021; 31(5): 772. CrossRef - The GDF15-GFRAL Pathway in Health and Metabolic Disease: Friend or Foe?
Samuel N. Breit, David A. Brown, Vicky Wang-Wei Tsai
Annual Review of Physiology.2021; 83(1): 127. CrossRef - Associations of GDF-15 and GDF-15/adiponectin ratio with odds of type 2 diabetes in the Chinese population
Xiaoying Wu, Wenting Xuan, Lili You, Hong Lian, Feng Li, Xiaoyun Zhang, Qingyu Chen, Kan Sun, Chaogang Chen, Mingtong Xu, Yan Li, Li Yan, Xiuwei Zhang, Meng Ren
Endocrine.2021; 72(2): 423. CrossRef - Decreased serum growth differentiation factor 15 levels after lifestyle intervention in patients with newly diagnosed type 2 diabetes mellitus
Xingxing He, Jiaorong Su, Xiaojing Ma, Jingyi Lu, Yufei Wang, Jun Yin, Yuqian Bao, Gang Hu, Jian Zhou
Obesity Medicine.2021; 24: 100345. CrossRef - The anti-diabetic effects of NAG-1/GDF15 on HFD/STZ-induced mice
Pattawika Lertpatipanpong, Jaehak Lee, Ilju Kim, Thomas Eling, Seung Yeon Oh, Je Kyung Seong, Seung Joon Baek
Scientific Reports.2021;[Epub] CrossRef - The Regulation of Circulating Hepatokines by Fructose Ingestion in Humans
Michael M Richter, Peter Plomgaard
Journal of the Endocrine Society.2021;[Epub] CrossRef - GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease
Dongdong Wang, Emily A. Day, Logan K. Townsend, Djordje Djordjevic, Sebastian Beck Jørgensen, Gregory R. Steinberg
Nature Reviews Endocrinology.2021; 17(10): 592. CrossRef - High Fat, High Sugar Diet and DJOS Bariatric Surgery Influence Plasma Levels of Fetuin-B, Growth Differentiation Factor-15, and Pentraxin 3 in Diet-Induced Obese Sprague–Dawley Rats
Jakub Poloczek, Monika Tarnawska, Elżbieta Chełmecka, Piotr Łaszczyca, Janusz Gumprecht, Dominika Stygar
Nutrients.2021; 13(10): 3632. CrossRef - Serum growth differentiation factor 15 level is associated with muscle strength and lower extremity function in older patients with cardiometabolic disease
Kazuhito Oba, Joji Ishikawa, Yoshiaki Tamura, Yasunori Fujita, Masafumi Ito, Ai Iizuka, Yoshinori Fujiwara, Remi Kodera, Ayumi Toba, Kenji Toyoshima, Yuko Chiba, Seijiro Mori, Masashi Tanaka, Hideki Ito, Kazumasa Harada, Atsushi Araki
Geriatrics & Gerontology International.2020; 20(10): 980. CrossRef - Growth differentiation factor 15 (GDF-15) is a potential biomarker of both diabetic kidney disease and future cardiovascular events in cohorts of individuals with type 2 diabetes: a proteomics approach
Axel C. Carlsson, Christoph Nowak, Lars Lind, Carl Johan Östgren, Fredrik H. Nyström, Johan Sundström, Juan Jesus Carrero, Ulf Riserus, Erik Ingelsson, Tove Fall, Johan Ärnlöv
Upsala Journal of Medical Sciences.2020; 125(1): 37. CrossRef - Elevated Plasma Growth and Differentiation Factor 15 Is Associated With Slower Gait Speed and Lower Physical Performance in Healthy Community-Dwelling Adults
Richard D Semba, Marta Gonzalez-Freire, Toshiko Tanaka, Angelique Biancotto, Pingbo Zhang, Michelle Shardell, Ruin Moaddel, Luigi Ferrucci, Anne Newman
The Journals of Gerontology: Series A.2020; 75(1): 175. CrossRef - Circulating Cardiac Biomarkers in Diabetes Mellitus: A New Dawn for Risk Stratification—A Narrative Review
Alexander E. Berezin, Alexander A. Berezin
Diabetes Therapy.2020; 11(6): 1271. CrossRef - Deterioration of Sleep Quality According to Glycemic Status
Myung Haeng Hur, Mi-Kyoung Lee, Kayeon Seong, Jun Hwa Hong
Diabetes & Metabolism Journal.2020; 44(5): 679. CrossRef - Effects of plant and animal high protein diets on immune-inflammatory biomarkers: A 6-week intervention trial
Mariya Markova, Liselot Koelman, Silke Hornemann, Olga Pivovarova, Stephanie Sucher, Juergen Machann, Natalia Rudovich, Ralph Thomann, Rosemarie Schneeweiss, Sascha Rohn, Andreas F.H. Pfeiffer, Krasimira Aleksandrova
Clinical Nutrition.2020; 39(3): 862. CrossRef - Effect of sleeve gastrectomy on plasma growth differentiation factor-15 (GDF15) in human
Ponnie Robertlee Dolo, Libin Yao, Peng Peng Liu, Jason Widjaja, Song Meng, Chao Li, Xiaocheng Zhu
The American Journal of Surgery.2020; 220(3): 725. CrossRef - Prognostication of clinical outcomes in diabetes mellitus: Emerging role of cardiac biomarkers
Alexander E. Berezin
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(2): 995. CrossRef - Effect of bariatric surgery on plasma GDF15 in humans
Maximilian Kleinert, Kirstine N. Bojsen-Møller, Nils B. Jørgensen, Maria S. Svane, Christoffer Martinussen, Bente Kiens, Jørgen F.P. Wojtaszewski, Sten Madsbad, Erik A. Richter, Christoffer Clemmensen
American Journal of Physiology-Endocrinology and Metabolism.2019; 316(4): E615. CrossRef - Regulation of Systemic Glucose Homeostasis by T Helper Type 2 Cytokines
Yea Eun Kang, Hyun Jin Kim, Minho Shong
Diabetes & Metabolism Journal.2019; 43(5): 549. CrossRef - GDF15 reflects beta cell function in obese patients independently of the grade of impairment of glucose metabolism
M.H. Schernthaner-Reiter, B.K. Itariu, M. Krebs, M. Promintzer-Schifferl, T.M. Stulnig, A. Tura, C.H. Anderwald, M. Clodi, B. Ludvik, G. Pacini, A. Luger, G. Vila
Nutrition, Metabolism and Cardiovascular Diseases.2019; 29(4): 334. CrossRef - Growth differentiation factor 15: A novel biomarker with high clinical potential
Stéphanie Desmedt, Valérie Desmedt, Leen De Vos, Joris R. Delanghe, Reinhart Speeckaert, Marijn M. Speeckaert
Critical Reviews in Clinical Laboratory Sciences.2019; 56(5): 333. CrossRef - Hepatokines—a novel group of exercise factors
Cora Weigert, Miriam Hoene, Peter Plomgaard
Pflügers Archiv - European Journal of Physiology.2019; 471(3): 383. CrossRef - Association between MIC-1 and Type 2 Diabetes: A Combined Analysis
Jianan Lu, Yue Zhang, Xingxuan Dong, Jiawen Lu, Chen Zhang, Jieyu Liu, Qingzhou Yu, Haoyue Teng, Qingkui Yao, Jieyun Yin, Liqiang Qin
Disease Markers.2019; 2019: 1. CrossRef - The mitochondrial unfolded protein response and mitohormesis: a perspective on metabolic diseases
Hyon-Seung Yi, Joon Young Chang, Minho Shong
Journal of Molecular Endocrinology.2018; 61(3): R91. CrossRef - Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases
Ana Luisa Cardoso, Adelaide Fernandes, Juan Antonio Aguilar-Pimentel, Martin Hrabě de Angelis, Joana Ribeiro Guedes, Maria Alexandra Brito, Saida Ortolano, Giovambattista Pani, Sophia Athanasopoulou, Efstathios S. Gonos, Markus Schosserer, Johannes Grilla
Ageing Research Reviews.2018; 47: 214. CrossRef - Serum Meteorin-like protein levels decreased in patients newly diagnosed with type 2 diabetes
Ju Hee Lee, Yea Eun Kang, Ji Min Kim, Sorim Choung, Kyong Hye Joung, Hyun Jin Kim, Bon Jeong Ku
Diabetes Research and Clinical Practice.2018; 135: 7. CrossRef - Growth differentiation factor 15 predicts advanced fibrosis in biopsy‐proven non‐alcoholic fatty liver disease
Bo Kyung Koo, Sung Hee Um, Dong Soo Seo, Sae Kyung Joo, Jeong Mo Bae, Jeong Hwan Park, Mee Soo Chang, Jung Ho Kim, Jieun Lee, Won‐Il Jeong, Won Kim
Liver International.2018; 38(4): 695. CrossRef - Serum Growth Differentiation Factor 15 in Parkinson Disease
Xiaomei Yao, Dong Wang, Lei Zhang, Lingling Wang, Zhenxiang Zhao, Si Chen, Xiaotang Wang, Tao Yue, Yiming Liu
Neurodegenerative Diseases.2017; 17(6): 251. CrossRef - GDF15 deficiency exacerbates chronic alcohol- and carbon tetrachloride-induced liver injury
Hyo Kyun Chung, Jung Tae Kim, Hyeon-Woo Kim, Minjoo Kwon, So Yeon Kim, Minho Shong, Koon Soon Kim, Hyon-Seung Yi
Scientific Reports.2017;[Epub] CrossRef - Growth differentiation factor 15 as a predictor of adverse renal outcomes in patients with immunoglobulin A nephropathy
Ki R. Na, Yoo H. Kim, Hyo K. Chung, Min‐Kyung Yeo, Young R. Ham, Jin Y. Jeong, Koon S. Kim, Kang W. Lee, Dae E. Choi
Internal Medicine Journal.2017; 47(12): 1393. CrossRef - GDF15 contributes to radiation-induced senescence through the ROS-mediated p16 pathway in human endothelial cells
Hyejin Park, Chun-Ho Kim, Jae-Hoon Jeong, Myungjin Park, Kwang Seok Kim
Oncotarget.2016; 7(9): 9634. CrossRef - GDF-15 and Hepcidin Levels in Nonanemic Patients with Impaired Glucose Tolerance
Mehmet Muhittin Yalcin, Alev Eroglu Altinova, Mujde Akturk, Ozlem Gulbahar, Emre Arslan, Damla Ors Sendogan, Ilhan Yetkin, Fusun Balos Toruner
Journal of Diabetes Research.2016; 2016: 1. CrossRef - Cardiac‐Secreted Factors as Peripheral Metabolic Regulators and Potential Disease Biomarkers
Colleen M. Dewey, Kathryn M. Spitler, Jessica M. Ponce, Duane D. Hall, Chad E. Grueter
Journal of the American Heart Association.2016;[Epub] CrossRef - Determinants of growth differentiation factor 15 in patients with stable and acute coronary artery disease. A prospective observational study
Serdar Farhan, Matthias K. Freynhofer, Ivan Brozovic, Veronika Bruno, Birgit Vogel, Ioannis Tentzeris, Sabina Baumgartner-Parzer, Kurt Huber, Alexandra Kautzky-Willer
Cardiovascular Diabetology.2016;[Epub] CrossRef - Effect of Atorvastatin on Growth Differentiation Factor-15 in Patients with Type 2 Diabetes Mellitus and Dyslipidemia
Ji Min Kim, Min Kyung Back, Hyon-Seung Yi, Kyong Hye Joung, Hyun Jin Kim, Bon Jeong Ku
Diabetes & Metabolism Journal.2016; 40(1): 70. CrossRef - Association between Growth Differentiation Factor 15 (GDF15) and Cardiovascular Risk in Patients with Newly Diagnosed Type 2 Diabetes Mellitus
Min Young Shin, Ji Min Kim, Yea Eun Kang, Min Kyeong Kim, Kyong Hye Joung, Ju Hee Lee, Koon Soon Kim, Hyun Jin Kim, Bon Jeong Ku, Minho Shong
Journal of Korean Medical Science.2016; 31(9): 1413. CrossRef - Comparison of Transcriptome Between Type 2 Diabetes Mellitus and Impaired Fasting Glucose
Ying Cui, Wen Chen, Jinfeng Chi, Lei Wang
Medical Science Monitor.2016; 22: 4699. CrossRef - Letter: GDF15 Is a Novel Biomarker for Impaired Fasting Glucose (Diabetes Metab J2014;38:472-9)
Bo Kyung Koo
Diabetes & Metabolism Journal.2015; 39(1): 82. CrossRef - Relationship between hepcidin and GDF15 in anemic patients with type 2 diabetes without overt renal impairment
Jun Hwa Hong, Yeon-Kyung Choi, Byong-Keol Min, Kang Seo Park, Kayeon Seong, In Kyu Lee, Jung Guk Kim
Diabetes Research and Clinical Practice.2015; 109(1): 64. CrossRef - Response: GDF15 Is a Novel Biomarker for Impaired Fasting Glucose (Diabetes Metab J2014;38:472-9)
Jun Hwa Hong, Bon Jeong Ku, Minho Shong
Diabetes & Metabolism Journal.2015; 39(1): 84. CrossRef - Is GDF15 a Novel Biomarker to Predict the Development of Prediabetes or Diabetes?
Kyu Yeon Hur
Diabetes & Metabolism Journal.2014; 38(6): 437. CrossRef
- Effect of a 6-Week Carbohydrate-Reduced High-Protein Diet on Levels of FGF21 and GDF15 in People With Type 2 Diabetes
- Bone Mineral Density in Prediabetic Men (Korean Diabetes J 2010;34:294-302)
- Ju Hee Lee, Hyun Jin Kim, Bon Jeong Ku
- Korean Diabetes J. 2010;34(6):386-387. Published online December 31, 2010
- DOI: https://doi.org/10.4093/kdj.2010.34.6.386
- 2,925 View
- 29 Download
- Bone Mineral Density in Prediabetic Men
- Ju Hee Lee, Yun Hyeong Lee, Kyoung Hye Jung, Min Kyeong Kim, Hye Won Jang, Tae Kyun Kim, Hyun Jin Kim, Young Suk Jo, Minho Shong, Tae Yong Lee, Bon Jeong Ku
- Korean Diabetes J. 2010;34(5):294-302. Published online October 31, 2010
- DOI: https://doi.org/10.4093/kdj.2010.34.5.294
- 4,439 View
- 30 Download
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background There are many studies regarding the effects of insulin on bone metabolism and changes in bone mineral density (BMD) in the setting of diabetes. The effect of prediabetes on BMD is not known.
Methods A total of 802 men participated in the Korea Rural Genomic Cohort Study (in Geumsan County). According to the results of an oral glucose tolerance test, subjects were classified into normal, prediabetic, and diabetic categories. One hundred twenty-four subjects diagnosed with type 2 diabetes were excluded, leaving 678 subjects for the study inclusion. BMD was estimated with a quantitative ultrasonometer.
Results The average BMD T scores of normal and prediabetic subjects were -1.34 ± 1.42 and -1.33 ± 1.30, respectively; there was no significant difference in the BMD T scores between these groups. The BMD T score was inversely associated with age and positively correlated with body weight, body mass index, total cholesterol, low density lipoprotein cholesterol, and HbA1c. On multiple linear regression analysis, low density lipoprotein cholesterol was the only statistically significant variable for prediabetes (β = 0.007,
P = 0.005). On the stepwise regression analysis, age (β = -0.026,P < 0.001), the body mass index (β = 0.079,P < 0.001), and low density lipoprotein cholesterol (β = 0.004,P = 0.016) were significant variables for prediabetes.Conclusions There was no significant difference in the BMD T score between the normal and prediabetic subjects. Further studies are needed regarding the association of fracture risk and changes in BMD with the development of overt diabetes.
-
Citations
Citations to this article as recorded by- More Rapid Bone Mineral Density Loss in Older Men With Diabetes: The Osteoporotic Fractures in Men (MrOS) Study
Flavia Tramontana, Nicola Napoli, Stephanie Litwack-Harrison, Douglas C Bauer, Eric S Orwoll, Jane A Cauley, Elsa S Strotmeyer, Ann V Schwartz
The Journal of Clinical Endocrinology & Metabolism.2024;[Epub] CrossRef - Prediabetes and skeletal health
Catherine Lindsay, Albert Shieh
Current Opinion in Endocrinology, Diabetes & Obesity.2023; 30(4): 200. CrossRef - Serum levels of sclerostin in prediabetes and its correlation with bone mineral density
Ajay Chauhan, Manoj Kumar Bhakhar, Parul Goyal
Journal of Family Medicine and Primary Care.2023; 12(11): 2702. CrossRef - The risk of hip fractures in individuals over 50 years old with prediabetes and type 2 diabetes – A longitudinal nationwide population-based study
Ho Youn Park, Kyoungdo Han, Youngwoo Kim, Yoon Hwan Kim, Yoo Joon Sur
Bone.2021; 142: 115691. CrossRef - Bone health in diabetes and prediabetes
Silvia Costantini, Caterina Conte
World Journal of Diabetes.2019; 10(8): 421. CrossRef - Bone mineral density in obese children with prediabetes
Ala ÜSTYOL, Mehmet Emre ATABEK
Ege Tıp Dergisi.2018; 57(2): 94. CrossRef - The Prevalence of Osteopenia and Osteoporosis Among Malaysian Type 2 Diabetic Patients Using Quantitative Ultrasound Densitometer
Shaymaa Abdalwahed Abdulameer, Mohanad Naji Sahib, Syed Azhar Syed Sulaiman
The Open Rheumatology Journal.2018; 12(1): 50. CrossRef - An association between bone mineral density and anabolic hormones in middle-aged and elderly men with prediabetes
Michał Rabijewski, Lucyna Papierska, Paweł Piątkiewicz
The Aging Male.2017; : 1. CrossRef - Amplification of transcutaneous and percutaneous bone-conduction devices with a test-band in an induced model of conductive hearing loss
Marn Joon Park, Jae Ryung Lee, Chan Joo Yang, Myung Hoon Yoo, In Suk Jin, Chi Ho Choi, Hong Ju Park
International Journal of Audiology.2016; 55(11): 653. CrossRef - Osteoporosis in Men with Diabetes Mellitus
Claire Issa, Mira S. Zantout, Sami T. Azar
Journal of Osteoporosis.2011; 2011: 1. CrossRef - Response: Bone Mineral Density in Prediabetic Men (Korean Diabetes J 2010;34:294-302)
Ju Hee Lee, Hyun Jin Kim, Bon Jeong Ku
Korean Diabetes Journal.2010; 34(6): 386. CrossRef - Letter: Bone Mineral Density in Prediabetic Men (Korean Diabetes J 2010;34:294-302)
Chul-Hee Kim
Korean Diabetes Journal.2010; 34(6): 384. CrossRef
- More Rapid Bone Mineral Density Loss in Older Men With Diabetes: The Osteoporotic Fractures in Men (MrOS) Study
- The Effects of D-Chiro-Inositol on Glucose Metabolism in 3T3-L1 Cells.
- Kang Seo Park, Jae Min Lee, Bon Jeong Ku, Young Suk Jo, Seong Kyu Lee, Kyung Wan Min, Kyung Ah Han, Hyo Jeong Kim, Hyun Jin Kim
- Korean Diabetes J. 2008;32(3):196-203. Published online June 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.3.196
- 2,510 View
- 26 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The target of the treatment of metabolic syndrome and diabetes is an improvement of insulin resistance. D-chiro-inositol (DCI) plays a role in a phospholipid mediating intracellular insulin action. In the previous studies, the urine level of DCI were decreased in the diabetic animal with insulin resistance. Some clinical studies showed that DCI improved a glucose level and HbA1c. Therefore we studied the relationship between DCI and glucose metabolism, especially insulin resistance. METHODS: To investigate the mechanism of DCI affecting the glucose metabolism, we examined the effects of DCI on 2-deoxyglucose uptake, gene expression of adipocytokines and AMPK pathway by using RT-PCR and western blot in 3T3-L1 cells. RESULTS: Insulin-stimulated 2-deoxyglucose uptake increased in DCI-treated cells by about 1.2-fold (relative to the control) and was inhibited by phosphoinositide 3-kinase (PI3 Kinase) inhibitors (Wortmanin, LY294002) and AMPK inhibitor (STO-609). In Western blot analysis, it didn't show the difference of phosphorylation of Akt and AMPK between DCI-treated group and control in 3T3-L1 cells. However, DCI decreased the gene expression of resistin in 3T3-L1 cells. CONCLUSION: DCI may involve other pathway of insulin signaling, but not PI3 Kinase and AMPK signaling pathways and it may be useful in managing metabolic syndrome by improving insulin resistance through increasing glucose uptake and decreasing resistin relevant to insulin resistance. -
Citations
Citations to this article as recorded by- Variation of Pinitol Content for Domestic Legume Species in Korea
Seung-Min Seo, Yeon-Shin Jeong, Dhakal Krisna Hari, Dong-Hyun Shin, In-Jung Lee, Eun-Sook Park, Jeong-Dong Lee, Young-Hyun Hwang
Korean Journal of Crop Science.2011; 56(1): 50. CrossRef
- Variation of Pinitol Content for Domestic Legume Species in Korea
- The Plasma Adiponectin Levels in Patients with Newly Diagnosed Type 2 Diabetes.
- Ihn Suk Lee, Bon Jeong Ku, Young Kun Kim
- Korean Diabetes J. 2008;32(2):173-174. Published online April 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.2.173
- 1,811 View
- 18 Download
- The Plasma Adiponectin Levels in Patients with Newly Diagnosed Type 2 Diabetes.
- Ihn Suk Lee, Yun Jeung Kim, Jong Im Kim, Jea Hyung Park, Bon Jeong Ku, Kang Seo Park, Tae Yong Lee, Young Kun Kim
- Korean Diabetes J. 2007;31(6):507-516. Published online November 1, 2007
- DOI: https://doi.org/10.4093/jkda.2007.31.6.507
- 2,151 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Adiponectin is secreted from adipose tissue and plays an important role in the regulation of glycemia and insulin resistance. In this study, the relationship between adiponectin and the adiposity, body composition, insulin sensitivity, lipid profile were respectively examined in newly diagnosed type 2 diabetes. METHODS: Total 1993 were participated in the Korea Rural Genomic Cohort Study (Geumsan County). After a 12-hour overnight fast, all subjects underwent 75-g oral glucose tolerance test. 105 of those were studied as newly diagnosed type 2 diabetes. The body composition was analyzed by means of bioelectric impedance analysis and the insulin sensitivity was estimated by fasting insulin, HOMA-IR and QUICKI method, respectively. RESULTS: Adiponectin positively correlated with high-density lipoprotein cholesterol (r = 0.246, P < 0.05). Adiponectin inversely associated with waist circumference (r = - 0.408, P < 0.01), triglyceride (r = -0.274, P < 0.05), ferritin (r = -0.260, P < 0.05), visceral fat (r = -0.248, P < 0.05), high sensitivity C-reactive protein (r = -0.228, P < 0.05) and body mass index (r = -0.225, P < 0.05). In multiple linear regression, waist circumference and high-density lipoprotein cholesterol were analyzed as independent variables of serum adiponectin. CONCLUSION: Adiponectin concentrations were closely related to waist circumference in newly diagnosed type 2 diabetes.
- Change of Cardiac Function and NT-proBNP According to Degree of Albuminuria in Type 2 Diabetic Patients.
- Bon Jeong Ku, Jeong Hee Kim, Jin Ok Jeong, Eun Seok Jeon, Dong Hyun Seo, Jae Min Lee, Si Wan Choi, In Whan Seong, Young Kun Kim
- Korean Diabetes J. 2004;28(1):28-35. Published online February 1, 2004
- 1,083 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The prevalence of diabetes mellitus has increased recently. The morbidity and mortality of diabetic patients are mainly caused by chronic complications, especially cardiovascular events. N-terminal proB-type natriuretic peptide(NT-proBNP) is a neurohormone that is secreted from ventricular myocardium due to myocardial dilatation or pressure overload. NT-proBNP has prognostic value, and reflects cardiac function in patients with myocardial infarction or heart failure. This study was performed to evaluate functional changes of the heart, according to the degree of albuminuria and the prognostic value of NT-proBNP in type 2 diabetic patients. METHODS: 57 patients with type 2 diabetes were divided into three groups according to their degree of albuminuria, these being normal(below 30mg/day), microalbuminuria(30 between 300mg/day) and overt proteinuria(over 300mg/ day). The clinical parameters in each of the patients were evaluated, echocardiography performed and the levels of NT-proBNP checked, and compared between the three groups. RESULTS: Of the 57 patients with type 2 diabetes the male:female ratio of 32:25, with mean age, duration of diabetes and BMI of 55.8+/-10.1 and 11.3+/-8.2 years, and 23.2+/-4.0kg/m2, respectively. Twenty-eight patients showed normal(49.1%), 15 microalbuminuria(26.3%) and 14 overt proteinuria(24.6%). The age, BMI, diastolic BP and glycosylated hemoglobin showed no significant difference between the three groups. The duration of diagnosed diabetes was significantly longer, the systolic blood pressure and serum creatinine levels significantly higher and the serum hemoglobin significantly lower (p<0.05) in the overt proteinuria compared to the normal group. The duration of diabetes was significantly longer and serum creatinine levels significantly higher in the overt proteinuria than the microalbuminuria group(p<0.05). The echocardiographic data showed no difference among the groups. The NT-proBNP levels also showed no significant difference, but tended to be elevated toward albuminuria. CONCLUSION: The cardiac function and NT-proBNP levels showed no significant difference between each of the albuminuric groups. This study suggests that the degree of albuminuria is not a predictive factor for changes of the cardiac function and NT-proBNP levels

 KDA
KDA
 First
First Prev
Prev





